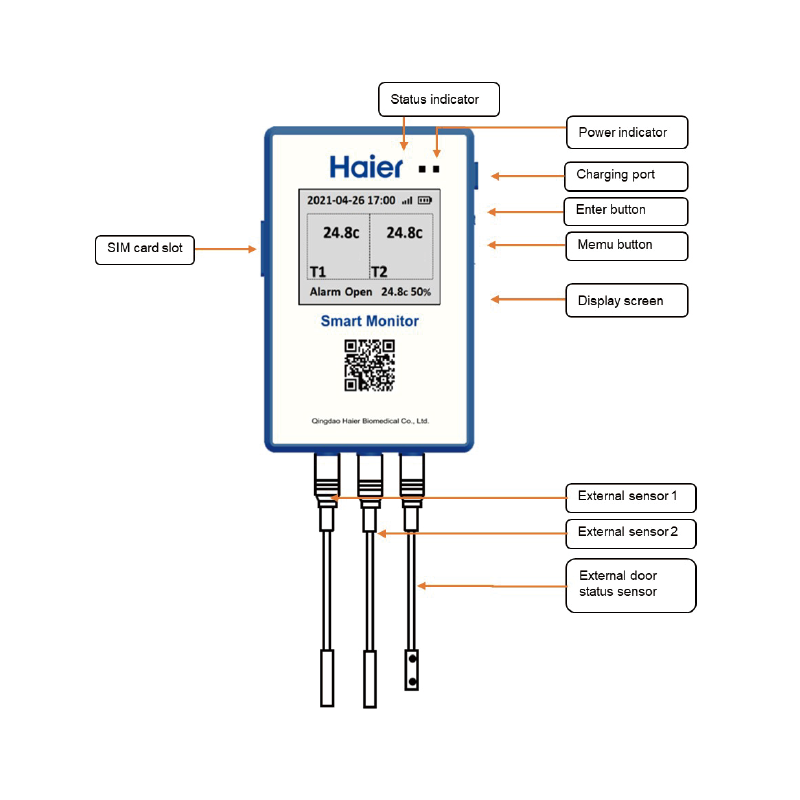
Haier Biomedical has achieved a total of 500 approved and pending patents, 300+ certified medical devices, with our solutions being used in over 160 countries’ reaching over 1Billion+ users. In China, our core R & D team have led or engaged in the drafting of 9 national and industry standards for the biomedical cryogenic storage industry, essentially covering for the sector all biomedical cryogenic storage products and solutions. Furthermore, Haier Biomedical participated in the drafting of World Health Organization (WHO) international standards, showing true leadership and development for the industry.


.png)























-800.png)

.png)